Bringing Gene Therapy to Life
Jonathan Thon, Ph.D. • December 11, 2023
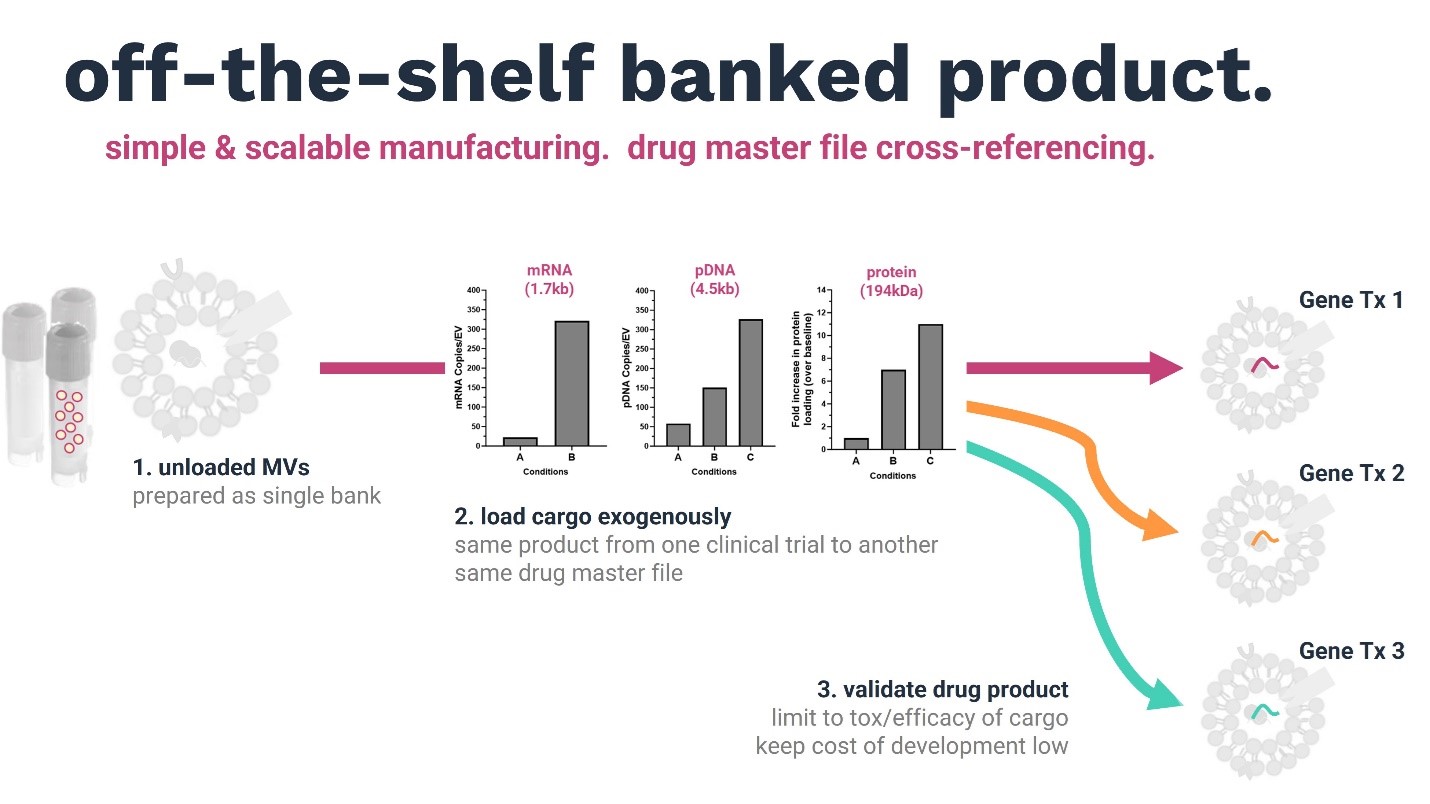
Our company’s key discovery is a non-viral gene delivery vehicle that can bypass the liver, overcoming the critical limitation of existing gene delivery systems. Our breakthrough is the simplicity, versatility, and scalability of our common manufacturing platform, which can be directed to the development of multiple drug products. Unloaded megakaryocyte extracellular vesicles (MkEVs) can be prepared as an off-the-shelf single bank, and diverse cargo/types (gene editors, RNAs, DNAs) loaded exogenously without affecting the characteristics of the delivery vehicle. Unlike viral and LNP vectors, MkEVs are not limited by carrying capacity and are innately immune privileged, enabling repeat dosing with no loss of efficiency due to immune reactions.
By keeping the delivery platform constant, we can leverage preclinical and clinical data from one application to another. The advantage is that the same MkEV product can be transferred from one clinical trial to the next using the same drug master file. Investors’ primary concern with cell and gene therapy approaches is the requirement for huge amounts of investment in preclinical studies before a company can hit its clinical proof-of-concept milestones. Our platform has the potential to limit the validation of cell and gene therapies to tox/efficiency of cargo, thus massively reducing the cost of development for partners.
In his keynote address at last year’s Meeting on the Mesa, Peter Marks, the Director of the Center for Biologics Evaluation and Research at the FDA, highlighted that:
“If we could get this paradigm to work, rather than having a manufacturer go back and do all of the preclinical toxicology and give us all the manufacturing information each time they submit something, they would just cross reference. That would allow us to focus on innovation that’s going to bring benefit to people.”
“We’d start by allowing an individual company to leverage the information from one application to another, and then, if that’s working well, we can consider expanding that concept further.”
We have the opportunity to do this.
The Advantages of Targeted Delivery
Gene therapy has a delivery problem. Delivery platforms aren’t specific – when injected into the blood, 90 to 95% go to the liver. And in most cases, immunogenicity and toxicity cap the number of doses to one.
Getting from Point A to Point B isn’t the only problem. Conventional vehicles for shuttling editors, nucleic acids, and other assets into target cells are limited in terms of the amount of cargo they can carry. And when it comes to delivering multiplex gene therapy assets to treat more complex diseases – it’s just not possible.
At STRM.BIO, we’re applying a non-viral platform using microvesicles derived from megakaryocytes to deliver gene therapies in vivo.
Megakaryocytes are cells in the bone marrow that make platelets. They also make microvesicles (extracellular vesicles that bud off the parent cell membrane); more than 80% of the vesicles in the blood are megakaryocyte-derived. They circulate in the blood and return to bone marrow where they can modulate the function of bone marrow stem cells. As such, they can serve as sentinels, with environmental changes influencing their biogenesis and composition, and therefore, their functional impact on bone marrow stem cells.
Here’s how microvesicles derived from megakaryocytes can transform the gene therapy landscape:
- They have very large carrying capacities and can be loaded with various types of cargo including RNA, DNA, protein, and multiplexed assets.
- Because they share the membrane and protein composition of their parent cells, they have cell surface molecules that drive very selective targeting to long-term hematopoietic stem cells in the bone marrow.
- They are innately immune privileged which means they can be dosed repeatedly.
Revealing First Platform Data at ASH 2023
Targeting
When these megakaryocyte-derived extracellular vesicles are injected into an animal, they biodistribute to bone marrow stem cells. When the cargo being carried is a messenger RNA coding for luciferase protein, and the vesicles are injected into mice, we observe biodistribution and luciferase protein expression in the bone marrow but none in the liver. When compared to a lipid nanoparticle with a messenger RNA coding for the same luciferase, the difference is stark–the majority of the LNPs are found in the liver. We can bypass the liver. These experiments were done with approximately tenfold less messenger RNA permouse, and a hundred-fold fewer vesicles injected into the mouse when compared to the LNP injections.
When the cargo is a pDNA coding for a red fluorescent protein or mCherry protein, we can track the fluorescent protein using plate reader and microscopy. The loaded extracellular vesicles pass through vascularized organs like the liver. They accumulate in the bone marrow and protein expression is almost exclusively in the bone marrow. When we collect the organs and analyze them with confocal microscopy, we can see protein expression in the bone marrow; there is some in the spleen which is a hematopoietic organ, but nothing in the liver.
When we translate those studies to a larger animal model, such as non-human primates, the biodistribution profile of the vesicles mirror that observed in mice. They accumulate in the bone marrow, with a small level in the spleen. When we load a pDNA into these vesicles and inject them into the non-human primates and quantify where that plasmid DNA is, we find that pDNA in the bone marrow. There is a small amount in the spleen, but nowhere else.
Repeat Dosing
In mice, the safety tox profiles have looked great. When we go into a non-human primate and we inject once a week for an extended period of time, the animals are clinically asymptomatic. Analysis of the blood and tissues shows no changes in blood cell counts, no evidence of kidney or liver damage, and no evidence of tissue inflammation.
A Focus on Fanconi Anemia
We believe there are many indications for which we can apply our delivery platform. Our initial focus is on blood diseases, and specifically, Fanconi anemia. Fanconi anemia is a rare genetic disorder affecting DNA repair. DNA damage accumulates, leading to bone marrow failure, blood cancers and other types of malignancies. Clinical studies have shown that expression of a normal Fanconi anemia protein will help address this phenotype.
The strategy that we’re proposing is to load a messenger RNA or a pDNA coding the normal protein into megakaryocyte vesicles and inject them into the bloodstream. Innate targeting of these vesicles drives biodistribution to the bone marrow and will enable expression of the wild type protein in bone marrow stem cells. This approach is not only a potential treatment for Fanconi anemia, but it can also be a way of priming patients to increase hematopoietic stem cells and blood cell counts before they undergo ex vivo gene therapy as well.
When we load a pDNA coding for Fanconi anemia protein and co-incubate Fanconi anemia knockout cells with these vesicles, we see Fanconi anemia protein expression. When we run a potency assay, we can see that the cells remain viable, so the Fanconi anemia protein that is being produced is functional.
VIDEO: STRM.BIO CEO Jonathan Thon presents at Cell & Gene Meeting on the Mesa (2023)
Broad Application of an Off the Shelf Process
Production of megakaryocytes is an off the shelf batch process. It doesn’t need to be matched to recipients. We source primary human hematopoietic stem cells from commercial suppliers, expand them, differentiate them into megakaryocytes, and from the megakaryocytes, harvest the extracellular vesicles. This becomes a bankable product, that we can thaw and load exogenously with cargo of interest. Once the drug product is loaded, we can store the product frozen. It can then be shipped to the site of treatment where it can be thawed and injected directly into the blood.
Importantly, the process of loading our extracellular vesicles doesn’t change their size or morphology. When we inject these vesicles into the blood, it doesn’t change their biodistribution profile or function.
Today, STRM.BIO is interested in a select number of diseases that we’re best empowered to pursue. But there are a lot more diseases that need to be treated, and potentially can be with this gene delivery platform. We’re actively discussing the use of our platform with gene therapy companies to translate them into in vivo success, and in doing so, reach a much larger population of patients in need of new treatments.