2023 Reflections and Outlook for 2024
Jonathan Thon, Ph.D. • January 26, 2024
The name STRM.BIO reflects our vision of what the world could look like if we’re successful in developing a platform that will bring gene therapy to life. We’d like to start 2024 by sharing with you a recap of the value we created in 2023, and a reminder of why it matters for the patients and families waiting for treatments and cures.
The heart of STRM.BIO’s value proposition is the use of microvesicles (MVs) as a safe, effective gene therapy delivery platform that permits repeat dosing. We have designed our platform to generate an off-the-shelf banked gene therapy delivery product that completely bypasses the liver and is compatible with diverse cargos and clinical indications. In so doing, we aim to democratize gene therapy by translating gene therapy programs to cures.
STRM.BIO’s value proposition rests on the advantages of our MV platform over other gene therapy delivery vectors, differentiated in 3 major ways:
1. STRM.BIO’s MV platform comprises an off-the-shelf banked product that is compatible with diverse cargos and clinical indications. Our simple, versatile, and scalable common manufacturing platform can be directed to the development of multiple drug products. Our product has consistent characteristics across multiple independent donors and batches, and following multiple freeze–thaw cycles.
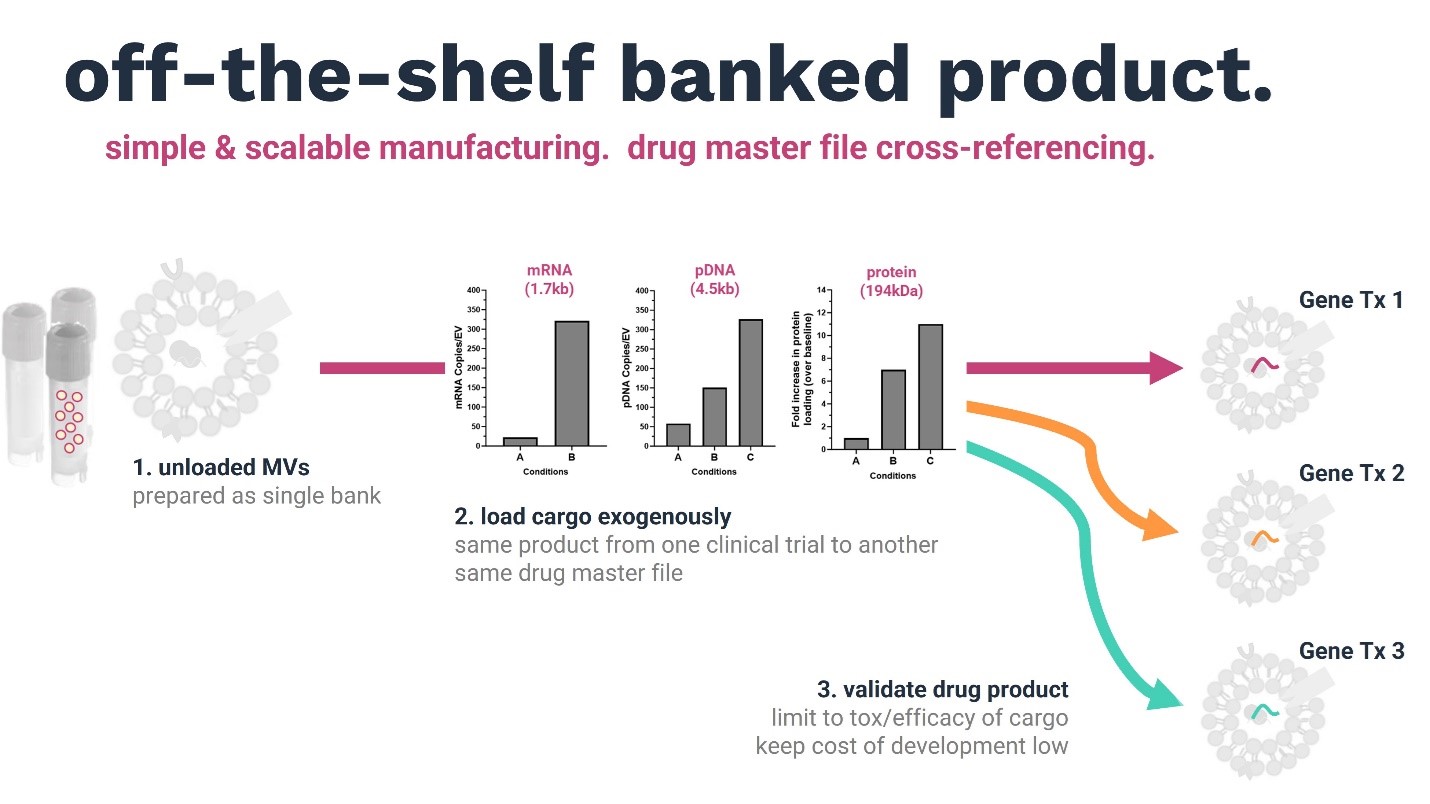
MVs can be prepared as an off-the-shelf single bank (stored frozen), and diverse cargo types (gene editors, RNAs, DNAs) can be loaded after thawing without affecting the functional characteristics of the delivery vehicle. By using the same delivery technology, we can leverage preclinical and clinical data from one application to another. The advantage is that the same MV product can be transferred from one clinical trial to the next using the same drug master file—a key industry need. Our platform has the potential to limit the validation of cell and gene therapies to toxicity studies and validation of the efficiency of the cargo, thus massively reducing the cost of development.
2. MVs completely bypass the liver and efficiently target bone marrow HSPCs in vivo. Crucially, our MVs have the innate ability to specifically home to and edit bone marrow HSPCs after injection into the bloodstream.
The incredibly positive read-outs from our mouse study in Q4-2023 showed cargo delivery/editing activity exclusively in the bone marrow. We saw preferential targeting of the hematopoietic stem and progenitor cells (HSPCs), entirely bypassing liver, lung, and spleen. This is a very big deal! The elegant and exquisitely sensitive Cre-LoxP reporter system we used for these experiments allows us to model the ability of MV-delivered gene therapy assets to edit the target gene in vivo by tracking the effectiveness of an MV cargo that edits a reporter gene so that it produces red fluorescence. Because the reporter gene is present in every single cell of the mouse, this system allows us to see exactly which tissues and cells undergo editing. In mice, the editing occurs at high levels in whole bone marrow but not at all in liver, lung, or spleen. Within the bone marrow, the editing occurs specifically in HSPCs, as identified by their expression of molecular markers that are unique to this cell type.
In vivo protein expression studies presented at ASH 2023 confirm that intravascular injection of our MVs bypasses the liver to preferentially target bone marrow HSPCs in both mouse and NHP animal models.
3. MVs are safe and permit repeat dosing. As human cell derivatives, our MVs have immune privilege, meaning that the body does not mount immune responses against them. In non-human primate trials we have confirmed a key aspect of our value proposition, namely that our MVs can be safely used for repeat dosing applications when needed. Following the successful repeat dosing of MVs in non-human primates, there was no detectable toxicity or inflammation as assessed by blood cytokine measurements and histopathologic tissue analysis.
genesis to-date
Our underlying strategy to date has proven sound despite serious market turbulence.
Our first strategic decision was the timing of our launch. The ideas behind STRM.BIO were conceived years before the company was actually founded, but the field hadn’t yet matured to match our ambitions. We were able to build on our ideas while the field progressed, and began our work to validate our value proposition in 2019, just as the window to overcome the delivery bottleneck was opening.
We chose to utilize a hybrid-virtual operating model right from the start, to leverage existing infrastructure and expertise rather than slowing our progress by reinventing the wheel. Potential investors were initially skeptical of this choice, but in retrospect turned out to be the right call: our model worked extremely well in the context of the COVID-19 pandemic, which began a few months after we launched. Having a hybrid-virtual model already up and running allowed us to leapfrog forward while other young companies were slowing down as they struggled to adapt to working in the new pandemic paradigm.
Our early success attracted attention and allowed us to recruit top thought-leaders in the field. We have been extraordinarily successful in assembling a world-class team and group of advisors, consultants, and collaborators.
The strategic and technical expertise and the consistent hard work of our talented team across all departments meant that we moved through the platform validation process faster than expected. In Q1 2022 were able to release a tranche of funding early to reach our next set of milestones faster, which allowed us to accelerate four key programs throughout that year: manufacturing scale-up, in vivo efficacy and dose-finding studies, non-human primate biodistribution, and clinical trial design. Our progress was further recognized in the form of substantial non-dilutive funding from the Bill and Melinda Gates Foundation (awarded in 2020, follow-on grant in 2023) and the NIH (Direct-to-Phase 2 SBIR grant awarded in 2022).
overcoming challenges
2023 began turbulently, with the company facing some challenges. One of our company’s key values, “collaborate to innovate”, became a central philosophy throughout the year. Collaboration helped us overcome everything from stubborn technical issues with in vivo protein expression to the unexpected Silicon Valley Bank failure, from which we emerged unscathed.
Our greatest challenge, though, has been the current significant decrease in new biopharma investment in the face of the global economic downturn. Anticipating a challenging market, we decided to raise additional funding in Q1 2023 to extend our runway into 2024. Our strong preclinical data and successful navigation of the market headwinds coming out of 2022 resulted in an oversubscribed seed extension round, which closed just 1 month after its launch. This decision contributed to our successes throughout the past year and would not have been possible without the support of the existing and external investors who participated in this financing.
Our investors’ confidence gave us the opportunity to hit critical value inflection milestones ahead of our Series A raise: protein expression in mice; biodistribution, repeat dosing, and tolerability in non-human primates; HSPC-specific editing activity in the Cre-LoxP system; and an FDA INTERACT submission. In the last year, we have successfully demonstrated proof-of-concept for the key differentiators of our technology.
In the last year, we have successfully demonstrated proof-of-concept for the key differentiators of our technology:
- In vivo biodistribution data from mice and non-human primates show that our MVs bypass the liver, lung, and spleen and deliver their cargo specifically to the bone marrow.
- Gene editing cargos delivered by our MVs in vivo preferentially edit long-term bone marrow hematopoietic stem and progenitor cells in mice, with no editing observed in the liver.
- Our MVs permit repeat dosing in non-human primates, with no apparent toxicity or inflammation.
- We are routinely producing our MVs at scale with consistently high quality. The MVs can be banked frozen, thawed, and loaded exogenously with diverse cargos (RNA, DNA, protein) without affecting their delivery characteristics.
The data outlined above were included in a well-received CATT meeting presentation and 2023 INTERACT submission to the FDA.
Nevertheless, the current tough market continues to affect us. In an effort to minimize risk, investors in our field have been focusing on later stages of therapeutic developmental, strongly favoring companies with clinical data already in hand. The preclinical stage companies that received funding in 2023 almost exclusively used bridge financing, extensions, add-ons, and insider rounds to meet the market’s new expectations for later phase data, and there is a trend of increased use of merger and acquisition deals as new investment activity remains depressed. We have therefore had to take some difficult short-term actions to safeguard our longer-term goals. We have streamlined our team to reduce our burn rate and extend our runway; we have prioritized platform over pipeline to facilitate earlier partnerships.
outlook into 2024
The decisions outlined above have not been easy, but they were the right thing to do. In this market, our success in making rapid progress through the platform validation milestones outlined above has put us in a better position than many young companies. As we look to 2024, the key technical asks from potential investors and partners mirror the next steps in our existing development plan and priorities. The resoundingly positive feedback on our data from potential investors and partners is facilitating the bridge financing we are on target to close for March of this year, which will extend our runway through this year, in preparation for our Series A in 2H 2024.
While the current market downturn is projected to extend through this year, there are signs that new capital is becoming available again, and that growth investors will step up their activity. Our success over the past year puts us in prime position to benefit from the first act of an upturn. We are excited for this next phase of STRM.BIO!
bringing gene therapy to life
To conclude, we have designed a platform-first approach to realizing our vision of an off-the-shelf banked product that allows for drug master file cross-referencing, with a simple and scalable manufacturing process. We have assembled an incredible team who have chosen to apply their talents to solving gene therapy’s delivery problem. We have generated meaningful proof-of-concept data that has created value by reinforcing the key differentiators of our technology, despite strong market headwinds, and without compromising our core values, our value proposition, or our vision.
We have put together an incredible, hyper-focused team who have chosen to apply their talents to solving gene therapy’s delivery problem. Together, we are delivering diverse gene therapy cargos. We are bypassing the liver. We are enabling repeat dosing. We are bringing gene therapy to life for the patients and families waiting for treatments and cures.
Because some things really matter.