A Better Way Series, Part II: The Pursuit of a New Solution
Jonathan Thon, Ph.D. • February 23, 2023
In Part I of this series, we explained why the current and final challenge facing gene therapy is the need for a better way to deliver gene editing technology into the right cells through a simple injection. At STRM.BIO we’re bringing gene therapy to life using a simpler, safer, and more practical delivery technology that we believe will help democratize the field and allow it to reach its full potential, bringing cures for multiple diseases within reach.
STRM.BIO is, of course, part of a diverse ecosystem of gene therapy research and development. Below is a brief overview of what microvesicles are and how they fit into the history and the current state of the art of the field.
Viral vectors: inspired by natural pathogens
The first gene therapy vectors harnessed the innate cargo delivery ability of viruses. Like gene therapies, viruses consist of DNA, RNA, and/or proteins that need to be delivered into specific cell types to be successful. These molecules are surrounded by a shell made of proteins, lipids (fats), and/or other components; the viral shell can interact with the target cell and transport its cargo inside. Modified versions of empty viral shells can be used to package gene therapies and deliver them into human cells. Different types of virus have evolved shells with a range of sizes, shapes, and molecular compositions, making viral vectors a versatile technology that continues to dominate the gene therapy delivery vehicle landscape today.
Synthetic nanoparticles: a safer version of viral vectors
Using vectors derived from pathogenic viruses is a convenient solution to the gene therapy delivery problem, but as you might expect this strategy also carries some risks and other disadvantages, which we’ll cover in more detail in the next installments of this series. After all, viruses didn’t evolve to be vectors for gene therapies.
Despite these limitations, many research teams are still working to improve viral vectors. However, other teams have switched to using synthetic nanoparticles made of lipids and other molecules. These particles are designed to reproduce the desirable properties of viral vectors in a safer format. Pfizer–BioNTech and Moderna both used lipid nanoparticles as the delivery vehicles for their mRNA-based COVID-19 vaccines, and very similar technology can be used to package and deliver small and medium-sized gene therapy cargoes. This technology is evolving rapidly, and research efforts in this domain are focused on improving delivery efficiency (which is lower than with viral vectors) without increasing toxicity.
Extracellular vesicles: harnessing the body’s natural inter-cell delivery process
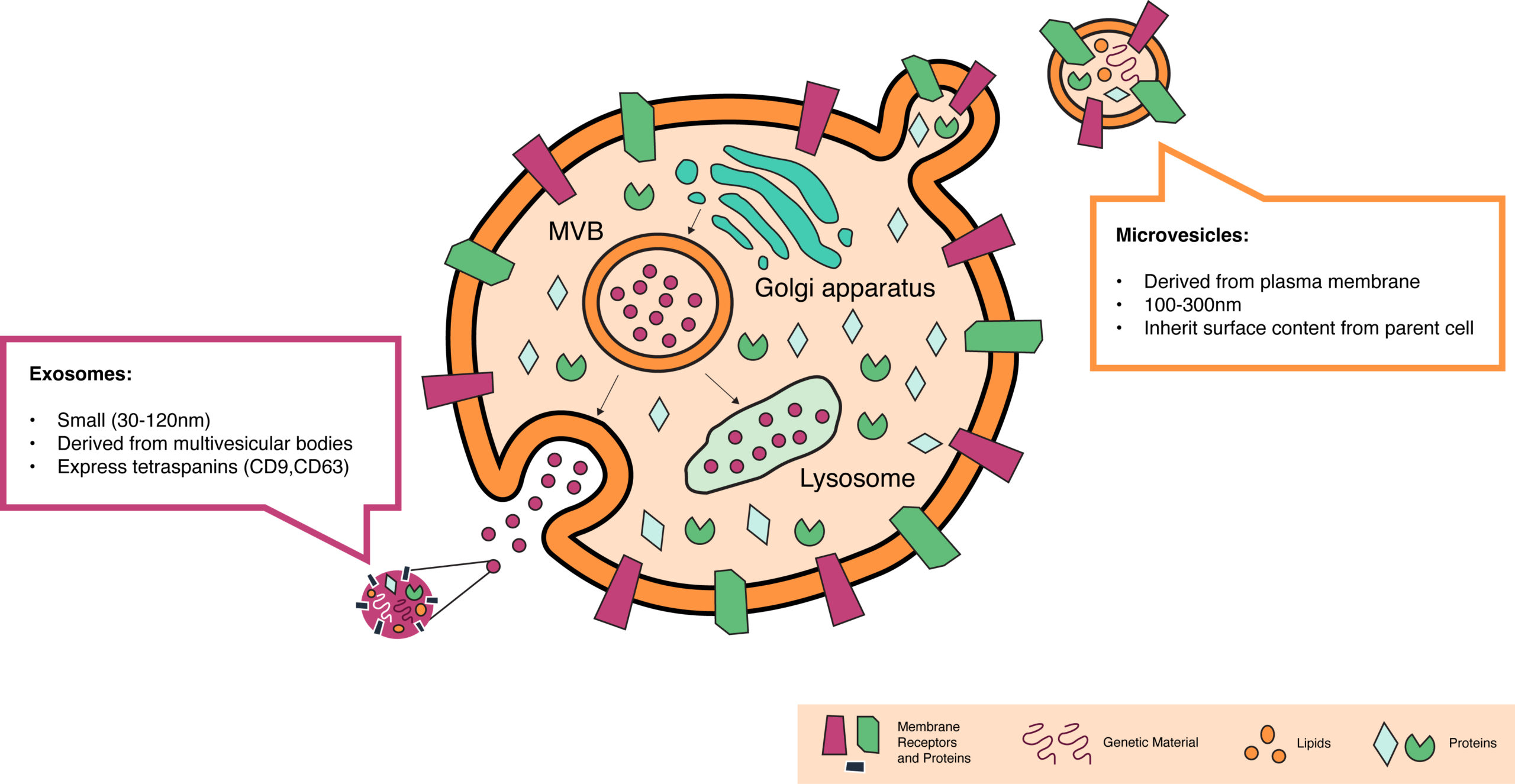
At STRM.BIO we are excited to be working on a highly promising category of delivery vehicles based on extracellular vesicles (EVs). EVs are lipid-based particles that are naturally secreted by almost all cell types in our bodies. They are the physiologic homolog to lipid nanoparticles (non-synthetic), and the way the body’s cells and tissues communicate with one another to accommodate environmental changes. They have an innate ability to encapsulate RNAs, DNAs, and proteins and to efficiently deliver this cargo into other cells—several orders of magnitude more efficiently than LNPs and other synthetic particles¹. EVs include exosomes, small vesicles that derive from inside cells, and microvesicles, which are larger and bud from the cell surface membrane (Figure 1). While we are capable of producing both, we have selected microvesicles as the delivery technology for STRM.BIO’s gene therapy candidates for reasons we will outline in our next post.
Microvesicles have many advantages over other current delivery technologies, and at STRM.BIO we believe that they have the potential to drive the final breakthrough that gene therapy has been waiting for. In the next post we’ll also explore how microvesicles stack up against viral vectors and synthetic nanoparticles in three key areas: cell targeting, safety and consistency, and dose repeatability.